Measuring Steric Impact: Understanding 'A Values'
- the-chemistry-editor

- Jul 18, 2024
- 5 min read
In Organic Chemistry, we often talk about bulky (sterically demanding) groups and substituents. But how do we quantify the relative bulk of one substituent compared to another? One method of comparing the steric demand of different substituents is by considering their 'A values'. These measurements are derived from studies on monosubstituted cyclohexanes and represent the energy cost of positioning a substituent in an axial conformation versus the preferred equatorial conformation.
In the field of organic chemistry, the concept of steric strain plays a crucial role in determining the stability and reactivity of molecules. One fundamental tool for comparing the steric demand of different substituents is the 'A value'. These values, derived from studying the steric strain in cyclohexane derivatives, allows the steric bulk of different substituents to be compared. This post delves into the significance of 'A values', how they are determined, and their implications in organic chemistry.
What are 'A Values'?
'A values' measure the steric strain experienced by a substituent when it occupies the axial position on a cyclohexane ring as opposed to the more favorable equatorial position. Essentially, the 'A value' represents the energy difference between these two conformations, expressed in kilocalories per mole (kcal/mol). This difference arises from 1,3-diaxial interactions, which are repulsions between the axial substituent and axial hydrogen atoms located on the same side of the ring. The higher the 'A value', the greater the energy cost of positioning the substituent in an axial orientation, thereby enhancing its effectiveness as a conformational anchor.

The Cyclohexane Ring: Axial vs. Equatorial Positions
Cyclohexane, a common ring structure in organic chemistry, can adopt a chair conformation that minimizes steric strain. In this conformation, substituents can occupy either axial (perpendicular to the ring) or equatorial (roughly parallel to the ring) positions. Axial positions are less favorable due to the steric clashes with hydrogen atoms on carbons three positions away, known as 1,3-diaxial interactions.
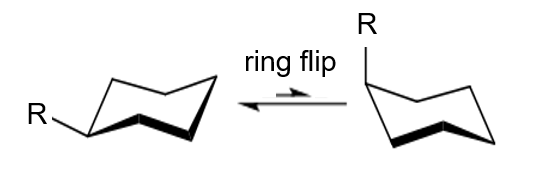
Determining 'A Values'
The 'A value' of a substituent is determined by measuring the energy difference between the axial and equatorial conformations of a substituted cyclohexane. This is typically done using experimental techniques such as NMR spectroscopy or computational methods. The higher the 'A value', the greater the steric strain experienced by the substituent in the axial position.

For example:
A methyl group (CH₃) has an 'A value' of 1.74 kcal/mol, indicating a 19:1 preference for the conformer with an equatorial methyl group over the one with an axial methyl group in methylcyclohexane.
Ethyl and isopropyl groups may appear to be significantly larger than a methyl group, but their steric demand is only slightly larger ('A values' of 1.8 and 2.2 kcal/mol). This is because these groups can still orient themselves such that a small hydrogen atom is directed over the face of the ring (additional energy cost is entropic).
A tert-butyl group (C(CH₃) has a much larger steric bulk and has to direct one of its three methyl groups over the face of the ring. This results in a very high 'A value' of 4.9 kcal/mol, meaning a 10,000:1 preference for the equatorially substituted conformer. Consequently, the tert-butyl group acts as a highly effective conformational anchor on cyclohexanes, even if this means placing other substituents on the ring in axial positions.

Importance of 'A Values' in Organic Chemistry
Predicting Conformational Preferences: 'A values' help chemists predict the preferred conformation of cyclohexane derivatives. Substituents with high 'A values' are more likely to occupy equatorial positions to minimize steric strain, influencing the molecule's overall shape and reactivity.
Designing Molecules: Understanding 'A values' aids in designing molecules with desired properties. For instance, in drug design, minimizing steric strain can enhance binding affinity and efficacy.
Stereochemical Outcomes: 'A values' play a role in determining the stereochemical outcomes of reactions involving cyclohexane derivatives. Knowing the preferred conformations can help predict and control the stereochemistry of reaction products.
Interpreting NMR Spectra: NMR spectroscopy can reveal information about the conformation of cyclohexane rings. 'A values' assist in interpreting these spectra by indicating the likely positions of substituents.
Implications for Multi-substituted Systems
'A values' are additive, allowing us to predict the preferred conformations of polysubstituted cyclohexanes. In the example below, the energy cost of placing a methyl group in an axial position is greater than the combined energy cost of placing two halogens in axial positions. Therefore, in this case the methyl group acts as a conformational anchor, and the preferred conformation features axial halogen atoms.

Important Considerations
When considering steric effects in molecular conformations, it's essential to account for the steric demand within a specific conformer rather than just the relative size of the substituent. Sometimes, a longer bond length can offset increased steric bulk. For example, trimethylsilyl (TMS, Si(CH3)3) and tert-butyl groups are similar in size, but the TMS group has a significantly lower 'A value' (2.5 vs. 4.9). This difference arises because C–Si bonds are considerably longer than C–C bonds, which reduces steric strain.
This principle also applies to halogens. Despite iodine being larger than bromine, and bromine larger than chlorine, these three halogens have similar 'A values'. The increasing bond length between carbon and the halogen as you go from chlorine to iodine compensates for the larger atomic size, resulting in comparable steric demands for each halogen substituent.
Aromatic rings, though they may appear bulky, have a planar structure and orient themselves to minimize 1,3-diaxial interactions. However, the 'A value' of a phenyl remains relatively large (3.0 kcal/mol) as its favored conformation now features clashes between the ortho-hydrogen atoms on the phenyl ring and the neighbouring equatorial hydrogens of the cyclohexane ring.
Hydroxyl (–OH) and methoxy (–OMe) groups exhibit relatively small 'A values' because the substituent on the oxygen can be oriented away from the ring. Substituents containing triple bonds, such as nitrile and acetylene, have low 'A values' (0.2 and 0.4 kcal/mol) because their linear structure imposes only a slightly greater steric demand than a hydrogen atom.
The 'A value' of a particular substituent should be treated with caution outside of regular cyclohexane systems, as the apparent steric effect may not hold true in other systems if electronic and stereoelectronic factors are also playing a role. For example, in cyclic hemiacetals (including sugars), heteroatomic substituents adjacent to the heteroatom prefer the more hindered axial position. In other systems, a 1,3-diaxial arrangement can become more favored than would be expected when hydrogen bonds can form across one of the faces of the cyclohexane ring.
Conclusion
'A values' are a crucial concept in organic chemistry, providing insight into the steric strain experienced by substituents in cyclohexane rings. By quantifying this strain, A values help chemists predict conformational preferences, design more effective molecules, and understand reaction outcomes. As we continue to explore and manipulate molecular structures, the understanding of 'A values' remains a fundamental aspect of organic chemistry, guiding us toward more efficient and targeted chemical synthesis.
References
Eliel, E. L., Wilen, S. H., and Mander, L. N. (1994). Stereochemistry of Organic Compounds. Wiley, New York.
Eliel, E. L., Allinger, N. L., Angyal, S. J., and Morrison, G. A. (1965). Conformational Analysis. Interscience Publishers, New York.




Komentarze